Applications
- Tube formation assays
- 3D cell culture
- Immunofluorescence staining
- Sprouting assays
- Live cell imaging of attached cells
Want to know if you should use a glass or a polymer bottom for your application? Find out here.
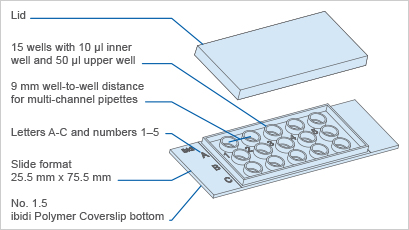
Specifications
| Outer dimensions (w x l) | 25.5 x 75.5 mm2 |
| Number of wells | 15 |
| Volume inner well | 10 µl |
| Diameter inner well | 4 mm |
| Depth inner well | 0.8 mm |
| Volume upper well | 50 µl |
| Diameter upper well | 5 mm |
| Growth area inner well | 0.125 cm2 |
| Coating area using 10 µl | 0.23 cm2 |
| Bottom: ibidi Polymer Coverslip | |

Define and print your experimental setup
Technical Features
- Standard slide format
- Closely fitting lid to reduce evaporation
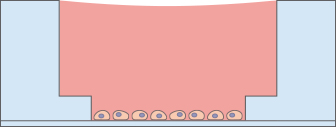
- 4 mm well within a 5 mm well
- Geometry enables pipetting of a homogeneous 0.8 mm thick gel layer*
- Provides homogeneous cell growth
- Compatible with staining and fixation
- Excellent optical properties for microscopy
- Compatible with multi-channel pipettes
- Made of biocompatible plastic material: no glue, no leaking
- Suitable for use with various types of gels* (e.g., Matrigel®, collagen, and agarose)
- Also available with a No. 1.5H glass bottom: µ-Slide Angiogenesis Glass Bottom
- Also available in a 96 well format: µ-Plate Angiogenesis 96 Well
*The gel matrix is not included with this product.
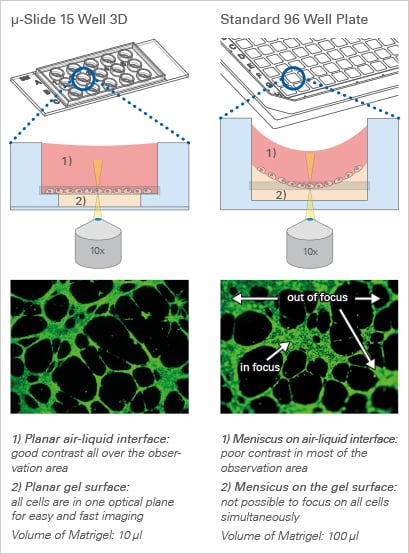
Every Cell in Focus:
The Principle of the µ-Slide Angiogenesis
The μ-Slide Angiogenesis has a specialized geometry for the easy, convenient, and reproducible conduction of tube formation assays. It is also ideal for sprouting assays, immunofluorescence staining, and general 3D cell culture.
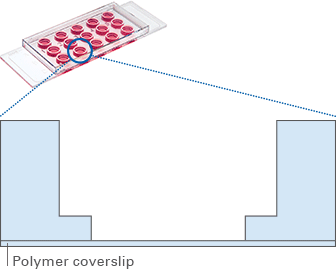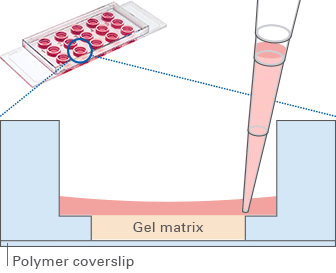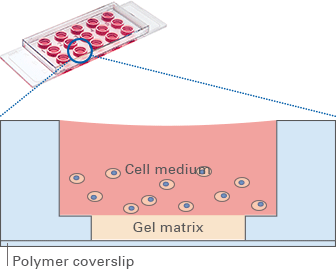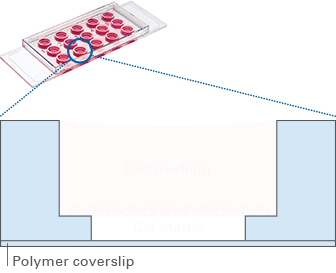
After the gel has been pipetted into the inner well and given time to solidify, the cells can be seeded on top of it for tube formation analysis. Due to the “well-in-a-well” technology, the amount of gel needed is reduced to only 10 μl per well, which is 10% of the amount used in regular multiwell plates.
Further, no gel meniscus is formed. This ensures the formation of a uniformly thick gel matrix on which all cells are in one optical plane, creating reproducible cell culture conditions. The µ-Slide Angiogenesis can be used with all common hydrogel matrices, such as Matrigel®, collagen gels, agarose gels, and hyaluronic acid gels.
With its 15 wells, the µ-Slide Angiogenesis is designed for low throughput assays. For large scale applications, ibidi provides the μ-Plate Angiogenesis 96 Well.
The µ-Slide Angiogenesis Can Be Used in a Wide Range of Applications
Tube Formation Assay
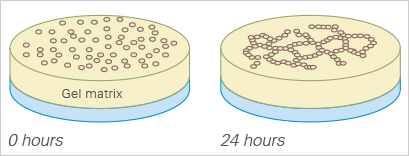
For a tube formation assay, endothelial cells are seeded on top of a 10 µl gel layer (basement membrane-like surface), where they form capillary-like structures. Typically, Matrigel® is used. The tube formation ability of the cells can be used to analyze the pro- or anti-angiogenic potential of various drugs.
Find more detailed information here:
For practical details, please watch the ibidi movie Performing an Angiogenesis Assay (MV 15). In our Application Notes, you will find detailed information on the setup, optimization, data analysis, and interpretation of tube formation assays:
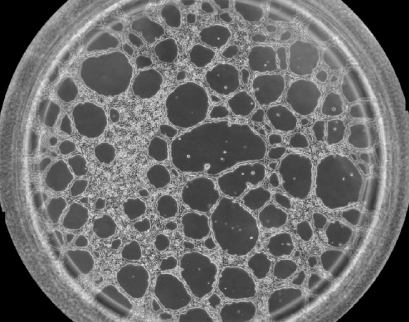
Phase contrast image showing one well of the µ-Slide Angiogenesis with HUVEC cells on Matrigel® after 12 hours of incubation during a tube formation assay.
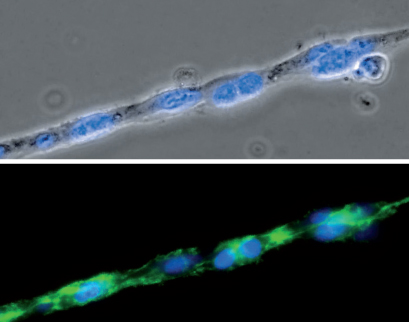
Phase contrast (top) and fluorescence microscopy (bottom) of a single strand composed of HUVEC cells during a tube formation assay in the µ-Slide Angiogenesis. The F-actin cytoskeleton is stained green and the cell nuclei are stained blue.
Sprouting Assay
The sprouting potential of a cell cluster can be conveniently analyzed using the µ-Slide Angiogenesis. To carry out a sprouting assay, spheroids or pieces of tissue (e.g., from the aorta), are placed onto or directly in the gel matrix before imaging.
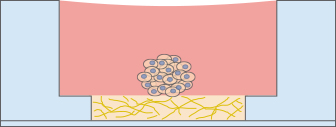
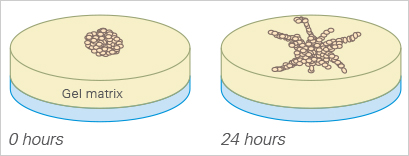
3D Cell Culture
In many cases, a 3D environment more closely resembles an in vivo situation than a 2D cell culture. The µ-Slide Angiogenesis is an easy and cost-effective solution for the cultivation and microscopy of cells embedded in gel matrices. The gel layer is directly connected to the medium reservoir above, which allows for fast and easy medium exchange by diffusion.
Besides cultures with only one cell type, the invasion behavior of different cell types (e.g., cancer cells and fibroblasts) can be investigated in a co-culture experiment.
Alternatively, a sandwich culture can be created using the same or different 3D gel matrices (e.g., Matrigel or collagen).
Also, non-adherent cells, such as blood cells or bacteria, can be immobilized for enhanced microscopy access.

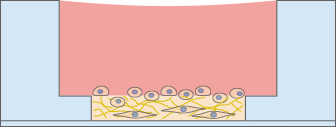
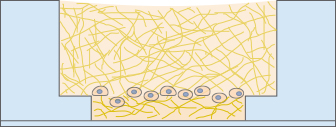
Immunofluorescence Staining
For analyzing the outcome of a tube formation or a 3D culture experiment, immunofluorescence staining can be performed in the µ-Slide Angiogenesis. The cells can be fixated and stained directly on or in the gel, then imaged using high-resolution microscopy.
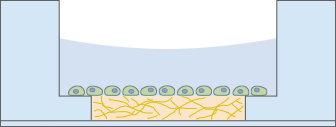
Focusing Cells
When using less than 10 µl of gel to fill the inner well of the µ-Slide Angiogenesis, small numbers of cells can be cultivated and focused on a soft gel surface.
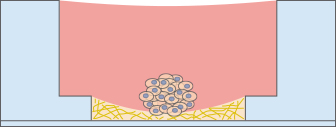
Low Volume Microscopy Chamber
The µ-Slide Angiogenesis can also be used as a low-volume microscopy chamber without any gel, which is useful for experiments where several wells with a low volume are required.